To maintain its size, a beef herd’s annual replacement rate typically equals the number of cows that exit annually due to death, marketing, or culling. A typical replacement rate of 15% has been suggested.
Throughout this calving season it is important to remember that continuing proper cow management is necessary for your cows to have a successful, tight calving window next year. One of the most effective ways to manage the post-partum interval is to maintain the body condition scores (BCS) of your herd.
In this video Michaela Clowser, Tammy Vaassen and Bill Halfman discuss the 2022 NCBA Quality audit results with dairy and beef producers.
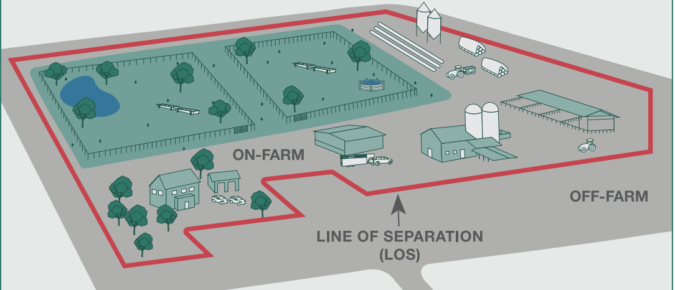
As the gavel falls on your cattle purchase, do you really know what you bought? Herd additions have inherent risk. Every movement of cattle onto your cow-calf operation—be they cows, heifers, calves, or bulls—brings biosecurity risks to your farm. It is critical to isolate new additions so that any sickness they break with is not shared with your home herd.
Winter feed costs typcially represent the largest portion of cow/calf expenses. A hay analysis is important to determine if hay will meet a cow herd’s nutrient requirements during winter. This factsheet will cover interpreting a hay analysis and to calculate winter feed needs.
A frequently heard recommendation for beef farms is to separate the 2-year-olds and thin cows from the main herd during the winter-feeding period. Three-year-olds may also benefit from being in this group because they are still growing. This is important every year, and likely even more important during years of limited forage resources.
Deworming decisions are farm-specific and depend on the age of the animal, how much exposure they had to infective larvae while grazing this spring and summer or the previous spring and summer, when they were last dewormed, and what products were used.
Management options that a spring-calving cow herd may consider to get through the winter feeding season when hay is in short supply. The examples given use general assumptions because options and costs vary from farm to farm and over time.
Bringing new calves to the feedlot is stressful for them due to transportation, adjusting to a new home, changing feed, exposure to disease, and establishing social order with new cattle. Minimizing both clinical and subclinical disease in feedlots is essential for producers to improve profitability.
Keeping your livestock safe from microbes, including bacteria, viruses, and fungi, is the biosecurity goal that all farms should have. Wearing clean, sanitized footwear helps meet this goal as foot traffic moves microbes to and around the farm.